Anything we experience at a sufficient intensity feels either pleasant or bad, potentially leading us to seek the stimulus out or remove ourselves from it. This basic assignment of subjective valence and by extension the valence we assign to possible future events, forms the basis of our emotional world. Emotional information is integral to decision-making processes, giving us motivation to act and choose a set of actions to bring us closer to our desired goals. Miss-regulation of emotional integration on the other hand is a hallmark of psychiatric disorders, leading to affect dysregulation.
While many of these concepts are intuitive from an interoceptive perspective, our knowledge of the etiology and pathophysiology of these disorders is strongly limited by our understanding of the non-pathological neuronal circuits underlying emotional processing which makes targeted intervention methods scarce.
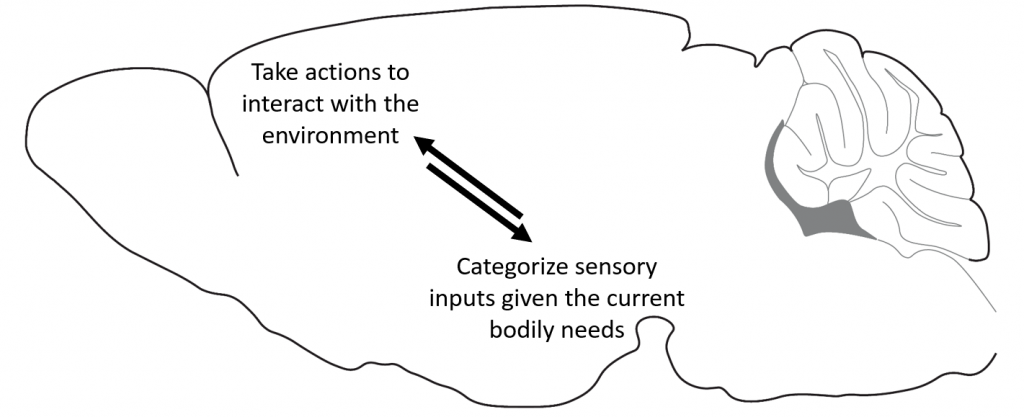
Two brain areas involved in valence assignment to environmental stimuli and integration of this information in decision-making are the basolateral complex of the amygdala (BLA) and the prefrontal cortex. My research interest is focused on characterizing the information transfer between these brain areas within the larger brain networks of decision-making and emotional processing in the mouse as a model system.
We are addressing this question by monitoring and manipulating the involved neuronal circuits during decision-making using in vivo and in vitro approaches such as electrophysiological recordings, optogenetic sensor imaging, and optogenetic actuator activation.
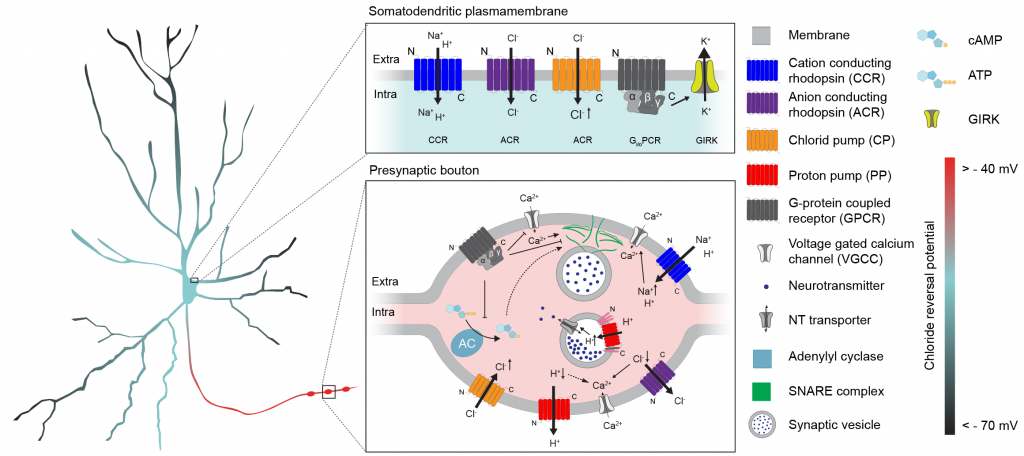
Optogenetics has enabled fantastic progress by increasing our precision in interacting with neuronal circuits at the relevant organizational levels. To overcome challenges in our ability to inhibit neurons, both at the level of action potential generation at the soma, as well as at the level of synaptic vesicle release, we have been working on designing new optogenetic tools. By designing soma-targeted chloride channels and light-gated G-protein coupled receptors, we enabled highly efficient somatic and presynaptic silencing.
Unmet needs, like pre- and post-synaptic specific interventions, continue to hold back neuroscience, motivating us to design new strategies for the next generation of optogenetic tools.